Nuclear Physics
Four new elements in the periodic table
Picture: Helmholtz
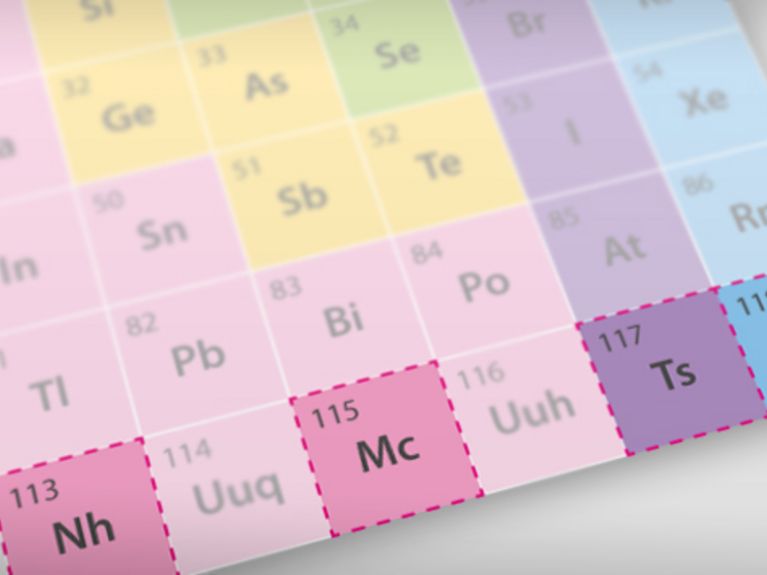
The periodic table of elements has now been expanded by four new additions. The international chemical union IUPAC have now officially consented to the introduction of the elements 113, 115, 117 and 118. Various research teams have succeeded in providing verification of the extremely short-lived atoms in recent years. But why are they looking for them in the first place?
The element 117 was first discovered by Russian research scientists at the Research Centre Dubna in 2010. Scientists at the GSI Helmholtzzentrum für Schwerionenforschung (heavy ion research) in Darmstadt were also successful in substantiating the element in 2014. In order to create it, they bombarded berkelium atomic nuclei (element 97) with calcium atomic nuclei ((Element 20). This generated a fusion process, and a new atomic nucleus – the super heavyweight 117 – saw the light of day, albeit for only a moment. Successful verification of the short-lived element was achieved by the research scientists through measurement of its radioactive decay. This resulted in daughter nuclei that have a significantly longer lifespan and were measured as well.
Readers comments